What is the technology and what is the intended use?
The Cyrcadia Health Circadian Biometric RecorderTM (CBR) system is a dynamic (time based) thermal differential detection system which detects abnormal circadian cellular temperature changes, including those which are associated with breast cancer. Temperature measurements are sequentially taken over time to determine incidences of circadian readings indicative of tumor infusion.
The iTBra™ consists of two flexible patches that are imbedded with thermistors and a recording/communication device connected to both patches. These are all applied under standard garments that are available in varying sizes to fit the majority of the population. An associated data transmission device gathers and transmits the raw data to Cyrcadia Health’s proprietary predictive analytic analysis software location for analysis under strict HIPAA protocol. Results are transferred back to the patient and physician for review and any further action. Though the device may be used on a general patient population as a monthly breast health monitoring alternative, it is particularly useful for those women with dense breast tissue.
Our detection system has shown to be tissue agnostic and has demonstrated equal response to circadian cellular change in all tissue types with all age groups scanned. Clinically, as evidenced by our flattened circadian profile over both breasts, our founders and principal investigators anticipate that beyond localization of thermal dynamic processes resulting from cancer cellular division, our circadian testing is also demonstrating the overall reduction in circadian cellular regeneration due to reduced Per1 and Per2 protein expression in the presence of familial or sporadic breast cancer. Both breasts react to the state of cancer from a circadian cellular perspective, even though cancer is present in only one breast. Therefore in the case of localized cancer in a specific quadrant of one breast, we display a flattened circadian cancer profile over both breasts further supporting the fact that both breasts are acting as a single gland to the infusion of the cancerous condition. Note descriptions and supporting study by the National Cancer Institute on Per1/Per2 reduced expression.
The question of earlier detection is interesting to our investigators as well. We were able to discern micro calcification infused cancers in dense parenchymal patients as demonstrated in our original Ohio State Study, where mammography missed these cancers all together. The circadian changes that produce heat, and reduced Per1 and Per2 proteins, begin with the initial cancer cell infusion and division. Our technology does not rely on specular reflection of an infused lesion of a particular size or degree of calcification to produce an image, but simply the active state of cancer cellular division to produce the reduced Per1 and Per2 protein effect as well as localized heat infusions discussed in the next paragraph.
In reference to our monitor, Dr. Farrar wrote in his summation following the initial clinical trial at The Ohio State University, “The term ‘false positive’ should be used with caution since the monitor was positive for three cancers missed by mammography which were either premenopausal or perimenopausal, with tumor sizes as small as 0.5 as well as micro-calcifications. Patient data which are positive on the monitor in the absence of mammographic or physical evidence of cancer does not preclude the presence of cancer at its earliest stages. These patients may be considered at “high risk” for the disease which may become clinically evident at a future date.”
Cyrcadia has further demonstrated that we have the ability to detect localized heat signature variations caused by cancer cell generation. Localized metabolic effects of Vascular Endothelial Growth Factor (VEGF) changes leading to neoangiogensis and aerobic glycolysis (Warburg effect) among other indications, increase the thermal signature in proximity to cancerous cell growth. This localized effect is the focus of our upcoming commercial evolution after further development and testing.
Our technology will have the capacity to notify the physician that the patient has a cancerous condition, as well as the coming ability to provide image display location of the lesion for physician review. We believe this advance in location guidance will be significant for the physician, especially with the interpretation required through utilization of mammography and ultrasound in this challenged patient condition. Visualization of the actual location will also lend higher credibility to the decision process for improved risk stratification of the BI-RADS4 population decision process for reduced non-cancerous surgical biopsy outcomes.
There are no known age or density related limitations to our device. Based upon our original trial results, we are targeting the following outcome for our upcoming clinical trial of 173 patients.
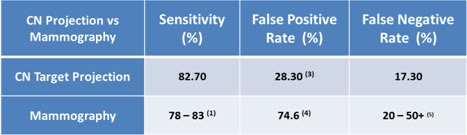
Sensitivity/ True Positive Rate = % of cancers correctly identified as cancer via biopsy
(1) 78% < age 50, 82%> age 50 on all tissue types – National Cancer Institute
(3) False Positive Rate = % of benign cases that were classified as malignant – OSU Monitor False Positive was 18% post neural net analysis, and could be potential early cancers not yet identified by Mammography
(4) False Positive Rate = % of non-cancerous patients biopsied by mammography indication – Breast Cancer.Org
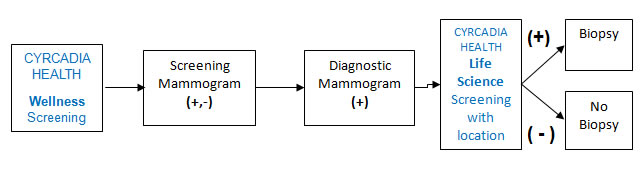
CYRCADIA HEALTH is releasing a two tier market structure, Life Sciences - Circadian Biometric RecorderTM (CBR) technology with both indication of circadian tissue profile associated cancer as well as location of suspected lesion for the hospital and clinical market. The Wellness - iTBra™ is intended for the over-the-counter and pharmacy models to supplement the woman’s monthly breast self-screening exam. Use Conditions are intended as follows:
Usage Scenario 1: CYRCADIA HEALTH patient flow from CYRCADIA HEALTH Wellness Monthly Self Breast Screening Exam (MBE) positive result leading to CYRCADIA HEALTH Life Science Biopsy Decision Assist - All Tissue Types
Usage Scenario 2: Monitoring of Dense Tissue Population supplementing or replacing Monitoring Mammography
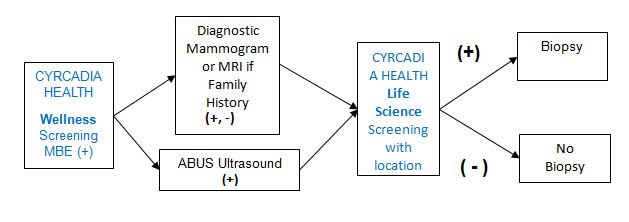
Usage Scenario 3: CYRCADIA HEALTH could be used in place of monitoring mammography as the primary screening mechanism for women diagnosed with Dense Breast Tissue
What is the tumor size/diagnostic resolution, demonstrated by the system and what is the target resolution?
Original Study Statistics – Monitor Identification:
Tumor size range identified by monitor: microscopic to 4.5 cms
Mean tumor size: 1.91cms
Age range of diagnosis: 26 to 69 years
Mean patient age: 53.2 years
Node neg. patients: 61%
Aneuploid tumors: 52%
Diploid tumors: 30%
(1) Ductal carcinoma: 65%
Other carcinoma: 22%
Original Ohio State University FWS Results
| Total biopsies: | 138 | ||
| Positive for cancer: | 23 | 138 | 17% |
| Total Detected | |||
| Cancers found on Mammogram: | 19 | 23 | 83% |
| Cancers found by FWS Monitor (Neural Net analysis) | 21 | 23 | 91% |
| Palpable Cancers: | 17 | 23 | 74% |
(1) Monitor found 15 ductal carcinomas and missed 3 carcinomas (all ductal) out of 23 carcinomas
(2) 3 cancers were found by the monitor that were missed by mammography. The subjects’ ages were 36, 38, and 44; the tumor sizes were 0.5, 0.7 and 2 cms. All patients identified by monitor and missed by mammography had dense parenchyma.
(3) 83% of all biopsies conducted in this study as a result of mammography referral were on non-cancerous lesions. Only 17% of all surgeries were on cancerous tumor conditions.
Which institutions are involved with upcoming clinical validation study?
The initial clinical studies had been completed at Ohio State University. Current and ongoing clinical studies are underway at El Camino Hospital, Mt. View CA and the original site at Ohio State University. Additional clinical trials are being evaluated. Other sites, such as the National University Hospital in Singapore and leading hospitals in India and Korea, have also expressed interest in limited validation studies.